octet rule definition
The Octet Rule is a general rule that is used to describe chemical bonding and draw Lewis Structures. The octet rule is a general rule of thumb that applies to most atoms.

Define Octet Rule Write Significant And Limitations O32d3vmm Chemistry Topperlearning Com
The octet rule is a rule that is sometimes broken.
. Lewis through a theory based on a cubical atoms. Hydrogen is excluded because it can. Octet rule noun A rule stating that atoms lose gain or share electrons in order to have a full valence shell of 8 electrons. Moreover the octet rule is a rule which is not very rigid and sometimes may get broken.
The octet rule states that an atom tends to have eight electrons in its outermost valence shell by forming covalent bonds through gaining or losing electrons from its outermost shell. A valence shell is. The octet rule dictates particular electron placement on the orbitals of the atoms nucleus. The octet rule is one of the chemical rules of thumb stating that atoms prefer to combine in a manner such that each atom has 8 electrons in their valence shells.
The rule states that Main Group elements form bonds in. When discussing the octet rule we do not consider d or f electrons. Exceptions to the Octet Rule In those cases where the octet rule does not apply the substituents attached to the central atom nearly always attain noble gas configurations The central atom does not have a noble gas configuration but may have fewer than 8 or more than 8 electrons. Elements look to lose or gain electrons in order to be like a noble gas.
Elements that obey octet rules are main group elements which are oxygen carbon nitrogen. The octet rule is an informal measure of how favorable a chemical bond is between atoms. The octet rule or octet rule is a postulate that is used in the context of the chemistry. The octet rule states that atoms gain or lose electrons to attain an outer shell electron configuration nearest that of a noble gas.
According to the Bohr model an atom consists of a nucleus of protons and neutrons orbited by a number of electrons. What Does Octet Rule Mean. Octet rule is called the chemical rule of thumb. The octet rule in chemistry is a chemical rule that explains the rules of atoms electrons and their forms.
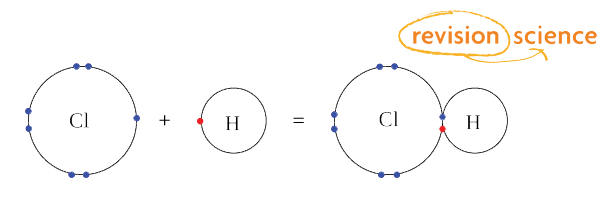
Why Elements Follow the Octet Rule Atoms follow the octet rule because they always seek the most stable electron configuration. The element attaining octet configuration has completely filled outermost s and p orbitals. It is the tendency that atoms show to complete their energy level with eight electrons to reach stability. Chlorine for example typically forms an anion with a charge of 1 -1 1 while sodium typically forms a cation with a charge of 1 1 1.
S-block and p-block elements obey octet rule except for hydrogen helium and lithium. The octet rule refers to an important chemical rule of thumb. This gives the atom a valence shell resembling that of a noble gas. When atoms have fewer than eight electrons they tend to react and form more stable compounds.
Atoms will lose gain or share electrons to achieve the electron configuration of the nearest noble gas 8 valence electrons except for He with 2. The octet rule was postulated by Gilbert Newton Lewis. The rule of octet refers to the filling of the s and p orbital with eight octet electrons in order to become stable like a noble gas s2 p6. It defines the propensity of elements belonging to the main groups in the periodic table to react with each other to form compounds and become stable.
What is the Octet Rule. The attractive force between atoms is informally measured with this rule. Octet rule It is a statement used in chemistry created by the chemical physicist Gilbert Newton Lewis which states that the tendency of the ions of the elements of the periodic system is to determine their final energy levels with eight electrons acquiring a stable configuration very similar to that of a noble gas. It states that every atom might want to have eight valence electrons in its outermost electron shell.
Moreover this rule means the observation that elements bond in a way such that every atom contains eight electrons in the valence shell. The octet rule in chemistry is the principle that bonded atoms share their eight outer electrons. The octet rule reflects the observation that the most stable ions of many elements have eight electrons in their valence shell for gaining the best possible stability. It was formulated by a man named Gilbert N.
Everything within our planet is made up of atoms and it. The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell.

The Octet Rule Help Definition And Exceptions Video Chemistry Ck 12 Foundation

Octet Rule Chemical Bonding The Octet Rule Is




Posting Komentar untuk "octet rule definition"